Baoji INT Medical Titanium Co., Ltd. was founded in 2003 by Mr. Zhan Wenge, who has more than 30 years of experience in the titanium industry.
After more than ten years of development, INT has grown into a benchmark enterprise in the R&D, production, and processing of medical titanium materials in the industry. Through more than 30 years of practical work in the research and development and production of titanium materials, INT people have gradually mastered the cutting-edge technology of titanium materials and the development trend of the titanium market, providing technical and equipment guarantees for medical titanium and precision die forging products.
 |
 |
 |
 |
 |
 |
 |
 |
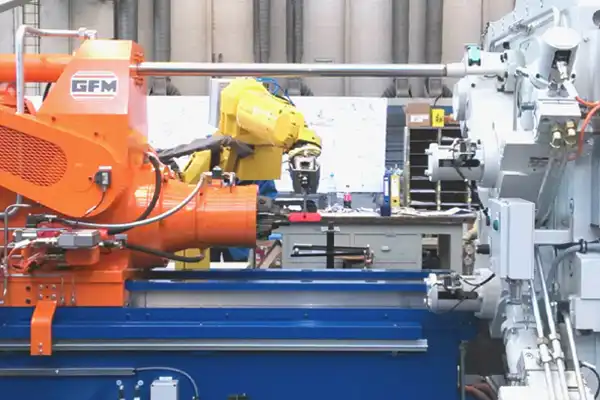
INT has profound experience in the medical titanium industry and provides high-quality and stable-quality medical titanium materials for customers to develop and use. We provide a full line of titanium material products for the medical field, including pure titanium, Ti6Al4V ELI titanium, and titanium alloy rods, wires, plates, and forged products in various specifications and sizes. INT has long been concerned about and well versed in the market development trend of titanium materials for medical applications, and invested in the establishment of Shaanxi Stand Biotechnology Co., Ltd. to achieve the goal of transforming scientific achievements into productivity. Stand began to become a leading professional manufacturer of precision die forging products such as metal joint handles at home and abroad.
 |
 |
 |
 |
 |
 |
 |
 |
After a long-term analysis of the titanium industry market, INT gradually visited titanium end product manufacturers and deeply understood their requirements and needs. At the same time, INT has steadily improved production, quality control, equipment verification, production management, etc. For a long time, it has been committed to customer service, product quality, and on-time delivery. Today, INT has developed many loyal partners and has established deep working partnerships with customers for more than 10 years.
Every product of INT has completely passed ISO9001:2015 international quality system certification, ISO13485:2016 medical device quality management system certification, and EU CE safety certification. The products and services provided include but are not limited to:
1. The field of clinical surgical implantation. High-precision and high-strength medical titanium and titanium alloy rods, wires, and plates are suitable for trauma, spine, nerve, and medical surgery fields. We specialize in turning and milling processes with complex processes and high precision.
2. The field of joints and prosthetic accessories. The introduction of advanced technology and equipment constitutes a complete production line. We are one of the professional manufacturers of precision die forging customized hip joints in the homeland and abroad. Providing joint stem blanks, joint stem finished products (OEM), dynamic hip screws (DHS), and prosthetic accessories, used in the field of surgical implantation and rehabilitation equipment.
3. Aerospace and automotive fields. Provide high-precision and complex die forging products, including automotive connecting rods, gearbox hollow shafts, aero-engine blades, etc.
4. Petrochemical field. Provide titanium and titanium alloy welded pipes and welding wires for petrochemical and natural gas fields and other industrial fields.
5. Electronics industry. Provide high-precision industrial titanium rods, wires, and plates for the manufacture of nuts and screws, circuit boards, mobile phone casings, and other electronic fields and other industrial fields.
6. Civil field. Design and develop a full range of titanium tableware, water equipment, and other outdoor products, registered trademark TAKIN and proud to provide customized services for customers. Committed to the development of a green economy and human health.
 |
 |
 |
 |
 |
 |
Company Culture
Vision
Leading the development of titanium technology, improving people’s life.
Mission
Leading Chinese medical titanium innovation, enhancing human welfare.
Management
Scientific planning, decision-making, and implementation.
Target
Liability-based, upholding integrity management, pursuing excellent quality, and enhancing customer satisfaction.
Company Certificates


Company History
1. Foundation Period. From Oct. 23th, 2003 to Dec. 31th, 2004.
Year 2003 - Founded by Titanium industry specialist Mr. Zhan Wenge, he is a titanium material industry professional with experience in R&D, production, and processing over 30 years, a deeper understanding of the medical titanium material industry marketing trends, and advanced technology.
Year 2004 - TIINT defined ourselves in Medical & Biomedical Titanium and Titanium Alloy Material R&D and production fields. Completed a serious of firm infrastructure. Obtained quality management system certification ISO9001:2000, covered the products and services of titanium and titanium alloy processed material for surgical implants, and titanium and titanium alloy bar.
2. Developmental Period. From Jan. 1st, 2005 to Dec. 31th, 2009.
Year 2005 - (1)Passed “The National Industrial Products Production License” certified.
(2)Set ultrasonic flaw detector and other inspection equipment and related monitoring equipment to enhance production quality control.
(3)Registered “TIINT” as TIINT’s trademark.
(4)Built new production plants, set forging workshop, rolling workshop, drawing workshop, and finishing workshop. Enhancing comprehensive production ability.
Year 2006 - Obtained “Proprietary Products Export & Import Right Certification” registered by Baoji City Customs.
Year 2007 - (1)Awarded “High-Tech Enterprise” approved by Shaanxi Provincial Science and Technology Agency.
(2)Obtained the projects' support of “Medical High Precision Titanium and Titanium Alloy Bar” and “Development of Medical High Precision Titanium and Titanium Alloy Material”.
3. Transferring Period. From Jan.1st, 2010 to Present.
Year 2012 - Obtained CE safety certification, reach the technical safety requirements specified in the EU and successfully open the European market. Providing product quality commitment European market.
Year 2013 - Purchased a new rolling machine, electric die forging machine, vertical microscope, and other production and testing equipment. Completed precision die forging production line, and gains the production ability of Titanium and Titanium alloy die forging hip joint, finished forging hip joint and prosthetic component, and others. Set a production line of Titanium alloy hip joints for surgical implants with 300,000 pieces annually. Created a basic condition of extension products for titanium high-precision bar. Actively engaged in new product development to produce high-quality automobile parts and aviation aircraft blades and other high-end products. Promoting the diversification of products, lay a good foundation for product range upgrades.
Year 2017 - Obtained ISO9001:2015 and ISO13485:2016 quality management system certification marked TIINT has more R&D capability and quality credit in the medical Titanium material production field while providing high-quality medical titanium material for more enterprises.
Year 2019 - Due to urban demolition, the production system was relocated from Xuguang Industrial Park in Baoji City to Bawanghe Industrial Park in Mei County.